Polyurea
Properties
Polyureas are reaction products of isocyanates and amines. These resin systems often compete with polyurethanes in similar coating applications. Like urethanes, they exhibit high flexibility, durability, and chemical resistance which are superior to those of most polyurethanes. The main difference between these two technologies is that amine terminated (-NH2) resins are used rather than hydroxyl teminated (-OH) resins. The reaction of the amines with isocyanates results in the formation of urea linkages (-NH-CO-NH-). The chemical structure of these polymers is given below.
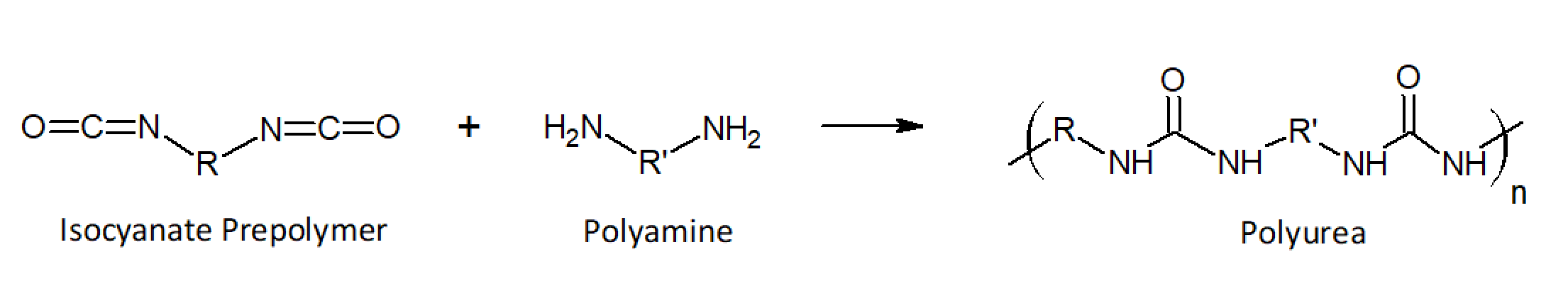
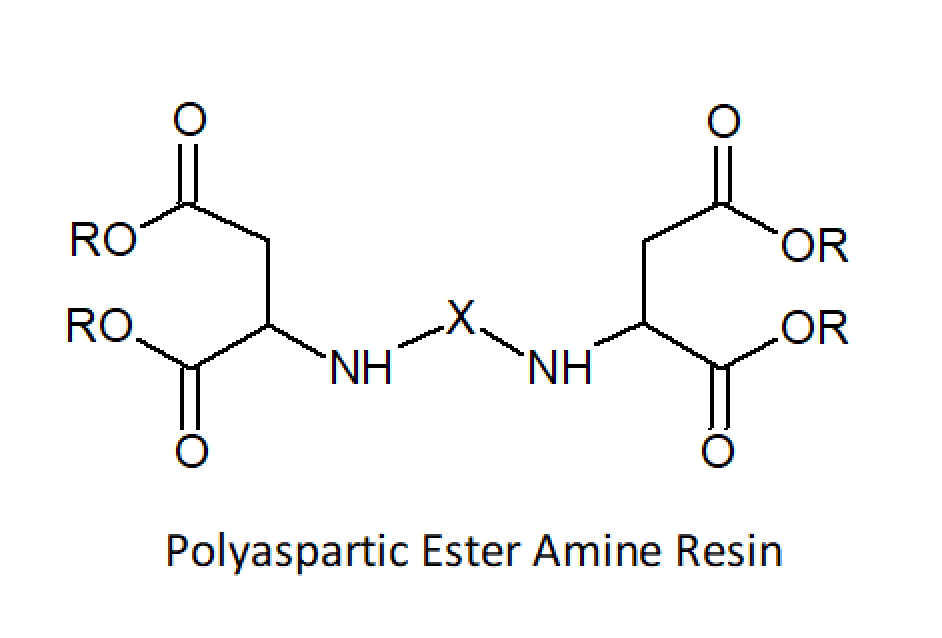
The isocyanate component is often a soft block-prepolymer based on aromatic or aliphatic isocyanates, such as toluenediisocyanate (TDI) and diphenylmethane diisocyanate (MDI) for aromatic systems and isophorone diisocyanate (IPDI) for aliphatic systems. The isocyanates are partially polymerized with high(er) molecular weight amines such as amine-terminated ethylene or propylene oxide based polyethers (Jeffamines®). Common chain extenders are low molecular weight amines such as diethylenetriamine (DETA), triethylene tetraamine (TETA) or etheramines for aliphatic ureas and diethyl-toluenediamine (DETDA), dimethylthio-toluenediamine (DMTDA), or N,N’-di(sec.butyl)- amino-biphenyl methane (DBMDA) for aromatic ureas. The latter two aromatic amines slow down the reaction significantly, whereas most aliphatic amines react rapidly with isocyanates, often within a few seconds. To slow down the reaction, aspartic ester amines are often used which provide secondary amine functionalities that cure noticeably slower than most other aliphatic amines.1
Ureas made with aliphatic isocyantes are more UV-stable and are less susceptible to oxidation and degradation than ureas made with aromatic isocyantes. However, they are more expensive and are only used when high UV stability is required such as for exterior coatings. For most other applications, aromatic isocyantes are preferred.
The majority of commercial polyureas are elastomers. The elastic network consists of alternating flexible polyether blocks and short and stiff urea blocks. The urea segments associate to hard domains that are connected via hydrogen bonds. These domains act as physical crosslinks and provide a restorative force when the thermoplastic urea elastomer is stretched. However, urea resins can also be chemically crosslinked, for example by reacting isocyanates with trifunctional amines such as polyoxypropylene triamine.
Polyureas are generally considered moisture insensitivity and exhibit good moisture barrier properties. Polyurethanes, on the other hand, are very sensitive to humidity, both during processing and while curing. The reaction with water produces carbon dioxide gas which leads to foaming and defects which can cause premature coating or adhesive failure. They also have higher heat resistance than comparable formulated polyurethane coatings. Another important advantage of amine/isocyanate reactions is fast and reliable cure even in the absence of a catalyst, whereas the cure of polyurethane coatings typically requires a catalyst and carefully controlled processing parameters such as temperature and humidity.
COMMERCIAL Urea Resins
A major manufacturer of commercial urea resins and spray coatings is Huntsman. The resins are available under the trade names:
- JEFFAMINE® (Polyetheramines)
- SUPRASEC® (Low-functional Isocyanate Pre-polymer)
- JEFFLINK® 754 (Aliphatic Chain Extender)
- UNLINK® 4200 (Aromatic Chain Extender)
Polyurea spray elastomer systems formulated with these ingredients are 100 percent two-component solids which are extremely reactive with cure times as short as 3-5 seconds.
APPLICATIONS
Polyureas have very low water permeability, which makes these resins ideal for moisture barrier applications. The most important application are industrial spray coatings and elastomers used in severe environments including exposure to hydrocarbons, salt water, diluted acids and bases, motor oils, and hydrogen sulfide gas. However, long term exposure to these chemicals may affect the performance and should be first tested. Most of the formulated polyurea spray coatings provide very fast cure even at temperature extremes of about 0°F and up to +250°-300°F.
1The ester groups attached to the secondary amines greatly reduce the reactivity compared to typical secondary amines due to the steric hindrance of these groups.